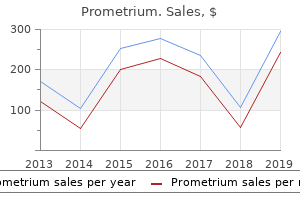
"Buy 200 mg prometrium with amex, 5 medications."By: Carlos A Pardo-Villamizar, M.D.
 https://www.hopkinsmedicine.org/profiles/results/directory/profile/0008959/carlos-pardo-villamizar
Purchase prometrium 100 mg with mastercardLevemir) is ready by a lower of the solubility of insulin such that insulin types a depot. In this formulation, insulin is stabilized on porous inhalable 2 �m to three �m fumaryl diketopiperazine crystals, with insulin adsorbed on the surface (Technosphere technology). The insulin particles have a excessive floor area, are deposited within the deep lung and dissolve within the lung, allowing insulin to diffuse across the alveolar epithelium. The formulation has a time to maximum plasma focus (tmax) of 15 minutes, and therapeutic ranges are maintained for 3 hours; hence the formulation is suitable for controlling postprandial glucose ranges and is superior, on this regard, to subcutaneous insulin, which has tmax of 2 hours. Long-acting peptides are required in certain disease states, similar to for the therapy of prostate most cancers through chemical castration. In this therapeutic routine, a luteinizing hormone releasing hormone agonist, goserelin, must be administered for several weeks to achieve its therapeutic effect. These elevated testosterone ranges ultimately create a adverse feedback loop within 14�21 days and ultimately diminished levels of testosterone are achieved, a process termed chemical castration. The greater level of the much less soluble lactide in the 3-month depot formulation ensures that matrix erosion/degradation and drug release occur at a slower price. Vaccines Introduction Vaccines are administered prophylactically to patients to defend against infectious illnesses. Mass immunization programmes at the start of the twentieth century coupled with access to clean water and the invention of antibiotics have had probably the most profound effect on human health ever witnessed. The innate immune response includes an activation of the immune system, the removing of foreign cells and proteins and an activation of the adaptive immune response. This early immune response offers a speedy and generic defence towards threats and is significant for the initiation of the adaptive and longer-lasting immune response, which leads to immunological memory and to the antigen-specific removal of the pathogen. The release of costimulatory molecules and cytokines as a half of the innate immune response also contributes to the activation of T cells. T-cell activation is adopted by B-cell activation and growth and the discharge of antibodies particular for the antigen. The mechanism by which vaccines stimulate an immune response has largely informed the development of vaccine delivery methods. The antigen is isolated from the cells by ultracentrifugation and chromatographic means. Delivery issues the foremost delivery problem surrounding vaccines is the maintenance of the chilly chain (from manufacture to administration) to prevent antigen degradation and guarantee potency. It can be fascinating to forestall undesirable bacterial development and to ensure a sufficiently high and extended immune response, as it will cut back the number of vaccination occasions. Delivery techniques Most multiple-use vaccines comprise a preservative such as the mercury compound thiomersal. Vaccines may also comprise antibiotics to stop unwanted bacterial contamination and stabilizers corresponding to 2-phenoxy esters. Furthermore, as stated already, all vaccines have to be stored in a continuous chilly chain to maintain antigen efficiency. This is a requirement, and this need for refrigeration contributes significantly to the costs of vaccine distribution and prevents low-resource communities from accessing vaccines. To circumvent the appreciable expense associated with the maintenance of a cold chain, researchers have prepared vaccine�sugar glasses during which a vaccine is blended with trehalose and sucrose and dried on a membrane to be hydrated when required. To produce vaccines with a chronic and enhanced immune response, huge efforts have been put into designing vaccine formulations. Immunopotentiators embody the imidazoquinolones, which have been proven to improve the immune response in preclinical studies, and the potassium aluminium salts. The virus is taken up by the cell and the viral genomic material enters the nucleus, where a number of copies of the virus are produced; the virus then infects other cells, and when viral titres are enough, the viruses are harvested. Alternatively, vaccine recombinant subunits are grown in host mammalian cells, by insertion of the gene for the antigen into bacterial, yeast or mammalian cells and the rising of multiple copies of the antigen. There are various progressive approaches to vaccine delivery, certainly one of which is the dry vaccine formulation mentioned earlier. A second innovation is intradermal vaccination utilizing microneedles; this provides the two advantages of painless delivery and the power to vaccinate large populations quickly. The pores fashioned on insertion of the microneedles into the epidermis quickly and painlessly reseal on withdrawal of the microneedles. Microneedles could additionally be fabricated from stable, hollow or dissolvable materials; dissolvable microneedles have been fabricated from maltose and amylopectin. These units, if adopted, may tremendously change the best way in which populations are vaccinated as they might be used for mass vaccination in the occasion of a pandemic, with sufferers vaccinating themselves in their own properties, having received the vaccines by publish. Genes are an info repository for the cell and organism, containing genetic data which is established at conception. Genes are normally faithfully copied in the course of the billions of cell division events that occur throughout a lifetime. However, a selection of ailments could also be traced to the mutation of varied genes and thus have a genetic foundation. Gene mutations will thus alter the resulting protein, and such alterations may result in illness. Disease-linked gene mutations, as in cystic fibrosis, lead to a nonfunctioning protein � the nonfunctioning cystic fibrosis transmembrane regulator. Theoretically, gene therapy could additionally be used to substitute a mutated gene and thus obtain a functioning protein. Proof of this therapeutic concept has been demonstrated in humans via the licensed gene medication Gendicine. Gendicine contains wild-type p53 to exchange mutated p53 in cancer cells and is delivered in an adenoviral vector. Delivery techniques the supply of genes in business gene therapies has so far been achieved with use of viruses, despite the very fact that one-third of the greater than 2000 gene remedy trials conducted to date used artificial (nonviral) vectors or no vectors at all. This replicationincompetent virus performs a mutation compensation perform and is administered intratumorally. The need for intratumoral injections is a severe limitation of the remedy because it is probably not easily used to deal with metastatic cancers. Patients receive one injection per week for 4�8 weeks, and every injection consists of 1012 viral particles in 1 mL of water for injections containing glycerol (to keep tonicity). Normally p53 is upregulated in cancer cells and causes cell apoptosis, antiangiogensis, an activation of an antitumour immune response and a downregulation of the expression of the multidrug resistance genes. On intratumoral administration of Gendicine, the adenovirus enters the most cancers cell through the Coxsackie virus and adenovirus receptor and the cell then begins to overexpress wild-type p53, causing apoptosis. The three accredited gene therapeutics (Gendicine, Glybera and Oncorine) are delivered by viral vectors. Gendicine, which is delivered in an adenovirus vector, is produced as a viral particle.
200 mg prometrium visaTo reduce leaching, the glass may be soaked in heated water or a dilute acid answer, which removes a lot of the surface-leachable salts. In addition to leaching of glass elements from the glass into the container contents, glass can undergo delamination, i. The components which affect delamination embrace the standard of the glass (discussed later), the character of the contents, the glass manufacturing and any sterilization processes. Pharmaceutical types of glass and containers Glass containers for pharmaceutical use are produced from either borosilicate (neutral) glass or soda�lime� silica glass. The containers are categorized in worldwide pharmacopoeias in accordance with their hydrolytic stability: the inside surface of the container or glass grains are made to contact water and the discharge of alkali ions from the glass into the water is measured. Plastics are additionally extensively used to package medicines in quite lots of methods, such as: � � � � � bottles for stable and liquid products; tubes for lotions, ointments and gels; pouches to contain individual suppositories; blister packs; baggage to include intravenous options and parenteral vitamin products; � overwraps; � bottle closures and closure liners; and � tamper-evident movies over bottle necks and stoppers. Advantages and limitations of plastics the use of plastics as a pharmaceutical packaging materials is growing because of the significant advantages and consumer desire. Plastics are gentle and shatterproof and could be clear or opaque (clarity may be desired for product inspection; opacity could also be desired to defend the contained medicine). Plastics are simply shaped and sealed, which supplies great versatility within the design of the pack, and permits the inclusion of administration aids, similar to a squeezable dropper. The many plastic materials, with a variety of physical, chemical, optical and efficiency properties, allows nice versatility. Plastics do, nonetheless, have sure disadvantages in contrast with conventional packaging supplies, such as glass and metallic, which limit their use. Plastics are less proof against heat and long-term gentle publicity in contrast with glass and metal. Plastics are also liable to undergo stress cracking, the place the presence of solvents, such as alcohols, acids or oils, causes a plastic pack to turn out to be brittle, crack and finally fail over time. Certain elements of the plastic packaging material can even leach out of the plastic and into the contained product. At the identical time, drug and excipients can adsorb to or take in into the plastic material. Polymers, macromolecules of repeating items known as monomers, are produced by addition or condensation reactions, the place one chemical species reacts with another (or itself) to type a new and larger compound. Thus polymers could also be copolymers (which encompass a couple of type of monomer) or homopolymers (containing only one type of monomer). For instance, ethylene may be polymerized into the homopolymer polyethylene, or can be made to react with a different species such as vinyl acetate to produce the copolymer ethylene vinyl acetate. The molecular weights of the person monomer parts in a polymer chain add up to the molecular weight of the polymer. Common plastic polymers utilized in pharmaceutical packaging have molecular weights ranging from approximately 10 000 to 1 000 000. Thermoplastic and thermosetting polymers Plastics may be divided into two lessons: thermoplastics and thermosetting plastics. In common, thermoplastics have linear and branched polymer chains, while thermoset polymers are cross-linked. At high temperature, thermoplastic polymers soften and turn into liquid, the polymer chains flow and the material could be moulded into quite so much of shapes, such as bottles, tubes and movies. Softening and reshaping by the applying of heat and mechanical pressure could be performed multiple times. Examples of thermoplastics embrace poly(vinyl chloride), polyethylene, polystyrene, polypropylene, nylon, polyester and polycarbonate. The properties and uses of essentially the most commonly used plastics in pharmaceutical packaging are shown in Table 46. The parison is reduce to the desired length, then two halves of the container mould close around it, the lower finish is sealed after which air is blown into the parison, inflicting it to increase to the form of the container. The completed, filled containers are removed from the filling tools, and extra plastic is removed. The capability to operate this course of in a managed setting with out the intervention of operators makes this process significantly suitable for aseptic production of sterile pharmaceutical merchandise (see Chapter 36 for more information). Further heating would lead to breakage of the bonds within the polymer and polymer degradation. Examples of thermoset polymers embrace urea formaldehyde, epoxides, urethanes, unsaturated polyesters and rubbers. These are primarily used to produce closures, as metallic coatings, and as adhesives within the pharmaceutical packaging business. Process residues and additives in plastics During polymer synthesis quite a lot of chemical compounds, corresponding to solvents, catalysts, initiators and accelerators, are needed to help the polymerization process. Unreacted monomers, process residues, additives and processing aids current in a plastic pack might leach out of the plastic material and into the packaged product. For this purpose, pharmaceutical grade polymers have strict limits on the quantity of chemical substances that may leach out of the plastic materials. Rubbers and elastomers Rubber has many applications in pharmaceutical packaging; for example, closures for vials and bottles, and ports on plastic luggage used to comprise parenteral vitamin merchandise. Elastomers are polymers that may be stretched (to greater than twice their unique length) and which return to their unique size as soon as the drive is removed. Elastomers could also be pure (extracted from rubber trees) or artificial (derived from petrochemicals), and customary pharmaceutical examples include butyl, chlorobutyl, pure and silicone elastomers. Rubbers are formulations of those elastomers, and along with the elastomer polymer, include a quantity of substances (2�10) corresponding to fillers, vulcanizing brokers, treatment accelerators, activators, plasticizers, lubricants, antioxidants and pigments. To produce rubber formulations, the elastomer and other required materials are placed in a mixer, which breaks the materials into small fragments and produces a uniform dispersion. The rubber is then trimmed and washed to remove residual materials that may have migrated to the surface throughout moulding. Residual materials can also be extracted from the rubber by different techniques, such as autoclaving. They are permeable to some extent to gases and moisture; they could additionally sorb parts of the packaged product, and residues and low molecular weight components could leach from them into the packaged contents. Other properties of rubber include its elasticity, hardness, stress to puncture, tendency to fragment, coring and resealability (following puncture with a hypodermic needle used to remove the contents), break pressure and vacuum retention. For rubber closures in vials/bottles, the rubber should be onerous sufficient to be agency yet allow simple insertion (and removal) of a needle through a vial closure. Appropriate elasticity will allow a great seal between the closure and the container, and allow resealing on removal of a hypodermic needle. To isolate the metal from the product, the steel floor is coated with vinyl-, acrylic- and epoxy-based resins. It has diverse purposes, including labels and leaflets, collapsible and rigid cartons, bags and sacks, and sachets. An exception is the sachet, where the paper is separated from the product by a layer of one other materials. Paper is outlined as a matted or felted sheet often composed of pure plant fibre.
Buy 200 mg prometrium with amexTemperature cycling research (from lower than zero �C to 40 �C) should be carried out for inhalation, nasal and transdermal merchandise, in addition to suspensions, creams and emulsions (even solutions, in some cases). Temperature fluctuations can encourage precipitation and/or particle growth, which can have a major effect on the standard and efficacy of those merchandise. The efficiency of inhalation and nasal merchandise at low temperature also needs to be characterized. Binary mixes of the energetic substance and excipient can then be used to confirm excipient compatibility. Binary mixes are normally a 1: 1 ratio of the energetic substance and excipient in either a powder blend/compact or an aqueous slurry combination (to examine the impact of moisture), although it could be more scientific for the ratio to mimic the degrees likely to be used in the formulation. Tertiary mixes, the place a further excipient is added to the binary combine, can also be used. Alternatively, thermal strategies, such as differential scanning calorimetry, can be utilized to detect interactions between the energetic substance and excipients. The producer must select the most acceptable kind of examine to achieve the utmost quantity of helpful info on the soundness of the drug product, whereas making the most efficient use of the formulation, manufacturing, analytical, stability cupboard storage, monetary and time sources at its disposal. Stability testing types a big part of a development programme each financially and in time, so it could be very important get it right first time. Pharmaceutical merchandise are likely to bear many modifications during their lifecycle. It is quite frequent for manufacturers to amend the drug product formulation or change the manufacturing course of and tools, change suppliers of active substances/ excipients, change the manufacturer of the drug product or change the container closure system. All these modifications might want to be submitted to the regulatory authorities and supported by stability research if a variation is sought to the advertising authorization. This requires producers to initiate an ongoing or rolling stability programme, by which at least one batch per 12 months of product manufactured, of every strength and every primary packaging type is included, except in any other case justified. The stability research design may be different from that used for licensing purposes, but should generate enough stability information to permit trend analysis in order that modifications within the stability profile can be detected. The stability of the product throughout transportation from the manufacturer(s) to the wholesaler(s) and eventually the pharmacy also wants be verified, as the beneficial storage advice applies to both static and cellular (during transportation) storage (Medicines and Healthcare merchandise Regulatory Agency, 2017). However, Schumacher (1972) and Grimm (1986, 1998) proposed reducing the variety of long-term take a look at conditions on the idea of the environmental situations in just four climatic zones. The current proposed meteorological criteria for the totally different climatic zones are described in Table forty nine. Climatic situations can differ between the totally different areas, and can change throughout the year. It is greater than the arithmetic mean temperature, and takes into consideration the Arrhenius equation. These storage conditions describe the final case for testing pharmaceutical merchandise. If the product is meant to be saved in a refrigerator, the long-term storage situation is 5 �C � three �C and accelerated storage is 25 �C/65% relative humidity, 30 �C/65% relative humidity or 30 �C/75% relative humidity. Products supposed for storage in a freezer have a long-term situation of -20 �C; merchandise that need to be stored below -20 �C are treated on a case-by-case foundation. Alternatively, a better storage humidity can be used, and water loss at the reference relative humidity could be derived via calculation. At forty �C, the calculated price of water loss during storage at not more than 25% relative humidity is equal to the measured water loss price at 75% relative humidity multiplied by three. Testing at accelerated and intermediate circumstances Accelerated situations are designed to be a reasonably extra stressful temperature and relative humidity setting than the long-term storage circumstances, with intermediate situations somewhere within the middle. Accelerated circumstances should be differentiated from stress testing, where more excessive situations could additionally be used. Pharmaceutical merchandise are typically stable (in the order of years) at long-term storage conditions, and thus stability testing over this era presents a sensible drawback for the producer. Testing at accelerated or intermediate circumstances can considerably reduce the time taken to generate stability information, giving an early indicator of the soundness of the pharmaceutical product. Accelerated or intermediate stability data can additionally be used in the extrapolation of the available long-term stability knowledge to set longer retest periods or shelf lives than the period covered by the long-term knowledge. It is for this reason primarily that it is suggested to provoke stability testing at accelerated and/or intermediate conditions, along with long-term conditions, in any formal stability research design. To scale back this uncertainty, the concepts of a moisturecorrected Arrhenius equation (Eqn. This overcomes a few of the limitations of the empirically (experimentally) derived Arrhenius equation being unable to accurately reflect the advanced individual molecular reactions concerned in degradation (as described earlier). For instance, in a stable dosage form, lively substance molecules can exist in an amorphous state or in crystalline domains, and could also be adjoining to different excipients. Consequentially, the molecules could degrade at completely different rates as a operate of the amount of degradation. Often, only a small amount of active substance is in a really reactive kind that will degrade, with the remaining being relatively steady. This exceeds the restrictions of the Arrhenius equation, and thus can make prediction of stability at other circumstances troublesome. If the quantity of lively substance converting to degradation products is saved the same, the proportions of the completely different reacting species stay the same (isoconversion), and this compensates for the complex reaction kinetics. There is an exponential relationship between relative humidity and drug reactivity, such that a small change in humidity will end in a large difference in stability and shelf-life prediction (Box forty nine. Long-term stability testing A manufacturer must make certain that long-term stability testing is conducted on the product, as intended to be marketed, at situations that characterize the beneficial storage situations throughout the retest period or shelf life. This guideline varieties an annex to Q1A(R2), and offers guidance on the basic testing protocol required to evaluate the sunshine sensitivity and stability of recent medicine and merchandise Stability Testing for New Dosage Forms (1996). This guideline extends Q1A(R2) for model new formulations of already permitted medicines and defines the circumstances beneath which decreased stability data may be accepted Bracketing and Matrixing Designs for Stability Testing of New Drug Substances and Products (2002). This guideline describes common ideas for reduced stability testing and supplies examples of bracketing and matrixing designs Evaluation of Stability Data (2003). This guideline extends Q1A(R2) by explaining potential situations where extrapolation of retest periods/shelf lives beyond the real-time knowledge may be appropriate. This document augments guideline Q1A, and offers with the actual aspects of stability take a look at procedures needed to take account of the special traits of products during which the energetic parts are typically proteins and/or polypeptides Q1B Q1C Q1D Q1E Q1F Q5C R signifies that a suggestion has been revised; therefore R2 signifies a second revision. Stability tips essentially describe how formal stability research on the active substance and the drug product should be carried out. Stability testing should be carried out on at least three primary batches of the pharmaceutical product in order to measure any batch-to-batch variability. In certain situations, such as the place the energetic substance is taken into account stable, the number of batches that must be included in a stability research could be reduced. The container closure system should simulate the commercial packaging for lively substances and must be similar to that used for the drug product. Liquid products packaged in containers with separate closures might need to be saved inverted and laid on their side to enable any interplay between the product and the container closure to be monitored. However, the ideas of bracketing and/or matrixing can be used to scale back the amount of testing. Matrixing is the place a selected subset of all the possible samples at a specific time point is tested. Reduced designs have pitfalls in that they might result in shorter shelf-life estimation, or the soundness studies have insufficient energy to detect some main or interplay effects.
Prometrium 100 mg onlineThe European Agency for the Evaluation of Medicinal Products publishes a decision tree for the choice of sterilization strategies. Steam (under pressure) sterilization Steam sterilization is the most dependable, versatile and universally used form of sterilization and depends on the mix of steam, temperature and stress. The typical cycle consists of a holding time of quarter-hour at a temperature of 121 �C at 15 psi 282 (103 kPa) gauge pressure (Table 17. Steam under pressure is commonly used except prohibited by lack of load penetration or warmth and/ or moisture injury. Steam can only kill microorganisms if it makes direct contact with them, so it is rather necessary to avoid air pockets in the sterilizer during a sterilization course of. For -ray irradiation, the method can take up to 20 hours, whereas for high-energy electrons (particle radiation), only a few minutes may be required. Pressurized cycle: at all times greater than atmospheric pressure; permits shorter contact time. Hence removal of air is an important a part of the method to guarantee effective sterilization. To remove the air present when an autoclave is loaded, autoclaves are equipped with air removal/displacement techniques. Noncondensable gases should also be removed and monitored; these are atmospheric gases corresponding to nitrogen and oxygen that type part of the initial atmosphere of the sterilizer. Other elements that have an result on the efficacy of steam sterilization are water content and steam purity. The optimal sterilization is obtained with saturated steam (as discussed in Chapter 16). Steam purity is set by the standard of the water, which can be affected by a selection of contaminants. Steam sterilization functions are informed/ regulated by numerous European and worldwide guidelines and requirements offering info on sterilizer design and installation, quality of steam, requirement for strain, development and validation and routine control, etc. Other procedures, such as sterilizing tunnels utilizing high-temperature filtered laminar air move or infrared irradiation to obtain speedy warmth transfer, are additionally available. Hot air ovens are often heated electrically and infrequently have heaters underneath a perforated bottom plate to provide convection currents (gravity convection type). Mechanical convection scorching air ovens are outfitted with a fan to assist air circulation and improve warmth transfer by convection (Joslyn, 2001). Dry warmth sterilization is inexpensive than steam sterilization and is efficient for the depyrogenation of containers/packaging. Overloading ought to be averted, wrappings and other limitations minimized and the load positioned to enable optimum air circulation. Dry warmth sterilization cycles are usually longer than for moist warmth sterilization, usually 2 hours at 160 �C (see Table 17. All warmth sterilization processes should embody heating up and cooling down periods. These extended periods at a raised temperature could improve the degradation of the product. Integrated lethality makes an attempt to examine the effects of heat on the inactivation course of throughout these intervals. Its calculation is complicated, and additional information can be found in the related pharmacopoeias. In practice, computer applications can be utilized to calculate the combined effect of entire processes, allowing a discount in the total process time. Gaseous sterilization the gaseous sterilization technique recommended by pharmacopoeias mainly employs ethylene oxide. The ethylene oxide sterilization cycle is advanced since many components have to be controlled over a long period (Table 17. In addition, ethylene oxide could be very flammable and might kind explosive mixtures in air. Gaseous sterilization utilizing ethylene oxide is however a well-liked sterilization process, primarily due to the low temperature used throughout sterilization, but in addition because of the amount of knowledge acquired on ethylene oxide sterilization processes through the years. The sterilized products have to be quarantined after the process to permit the removing of gas. The European Pharmacopoeia and different worldwide requirements set limits for ethylene oxide residue ranges. As with ethylene oxide, its sterilization cycle is somewhat complicated as several parameters need to be controlled (see Table 17. The becquerel (Bq) measures the activity of a supply of radiation (physical radiation). The electronvolt (eV) measures the energy of radiation and is normally expressed as hundreds of thousands of electronvolts (MeV). Gamma radiation is extremely penetrative, causes negligible heating of the sterilized product at regular doses and induces no radioactivity within the final product. Irradiation of a product could be carried out in batches however is extra commonly a steady process utilizing a conveyor system. The products move through the irradiation chamber and are irradiated from one or two sides. The source is shielded with concrete to protect the operators and the surroundings. For instance, one hundred mm of a product with a density of 1 g cm-3 would minimize back the cobalt-60 depth by 50%. A cobalt-60 source of 1� 1016 Bq to 4 � 1016 Bq is used for industrial irradiation, and this offers a radiation dose in extra of 25 kGy. When not in use, the radioactive supply is submerged in water for shielding and cooling. Particle radiation sterilization makes use of -particles which would possibly be accelerated to a excessive energy by application of high-voltage potentials (no radioactivity required). Their low power means that beams from particle accelerators are much less penetrating than -rays, with solely 10 mm of a 1 g cm-3 material being penetrated per million electronvolts (MeV). However, an important advantage of particle radiation sterilization is that the source can be turned off and is directional (Lambert, 2013). The design of an accelerator may be custom-made to explicit purposes by including different vitality and energy requirements. The beam supply is shielded with concrete and products are conveyed by way of the exposure space and irradiated. Another benefit is that shorter exposure times are required than these wanted for -ray irradiation. High-energy beams with energies of 5 MeV to 10 MeV are used for sterilization, the accelerating subject being generated using radiofrequency or microwave vitality. Once it has been accelerated to the required power, the beam of electrons is managed by magnetic fields which can alter its dimension, form or course (McDonnell, 2007). Validation of radiation sterilization includes using Bacillus pumilus as a organic indicator and dosimetric analysis (discussed later on this chapter). The routine monitoring involves measurements to be positive that all products are receiving the required dose. The radiation sterilization process is highly regulated, and there are a number of European and worldwide requirements and pointers available with information on requirements for the development, validation and routine management of the method.

Discount 200 mg prometrium overnight deliveryMucins are extremely large glycoproteins (up to 3 � 106 Da per monomer) with protein areas rich in serine and threonine that are linked, by their hydroxyl side teams, to sugar chains (O-glycosylation). They are anionic (negatively charged) as a end result of most of their terminal sugars include carboxyl or sulfate groups. Entanglement of mucin polymers results in the formation of a mucous gel and the era of a mesh which is stabilized by noncovalent calcium-dependent cross-linking of adjacent polymers. The sugar side chains bind massive quantities of water, allowing the mucus to act as a lubricant and a reservoir for the periciliary fluid inside which the cilia beat. Mucus is a viscoelastic gel with the properties of both a deformable stable (elasticity) and a viscous fluid (see Chapter 6). Cilia can transport mucus only of the appropriate viscoelasticity, and this is controlled by the level of mucus hydration. The presence of mucus at the epithelial floor of the nasal cavity offers a further potential diffusion barrier to drug supply. The ability of a molecule to diffuse through the gel is a operate of the scale of the drug molecule, the effective mesh measurement of the mucous gel shaped by the mucin molecules and any interactions between the drug and the elements of the mucous gel. The diffusion of small, uncharged molecules appears to be less affected by a mucous barrier than the diffusion of bigger, cationic molecules. Mucus seems to present a barrier to the permeation of small, relatively hydrophobic molecules corresponding to testosterone and that is believed to outcome from their interaction with the lipid component of the mucous gel or the hydrophobic (nonglycosylated) area of the mucin molecules. It is believed that such small molecules are solely able to form low-affinity, monovalent bonds with the mucins which persist for just a brief time. A variety of studies point out that positively charged (cationic), low molecular weight medicine, similar to amikacin, gentamicin, tobramycin and some -lactam antibiotics, bind electrostatically to negatively charged elements in mucus. It is believed that such molecules bind tightly and polyvalently to the negatively charged sugar residues of the mucins. Large positively charged nanoparticles, corresponding to these coated with chitosan, bind particularly tightly to mucous gels by an identical mechanism. In addition, proteolytic enzymes (proteases and aminopeptidases) present a possible barrier to the absorption of sure peptides. Drugs could also be metabolized in the lumen of the nasal cavity or as they pass across the nasal epithelium. However, the metabolic activity of the nasal cavity is less than that of the gastrointestinal tract (on a nanomole per milligram of protein basis) and, as nicely as, there are a variety of things that may have an effect on the relevance of metabolism to drug absorption. These include the quantity of drug applied to the nasal floor area, the chemical nature of the drug, the speed of elimination of the drug from the cavity and its price of absorption throughout the mucosa. Physicochemical properties of drugs affecting intranasal systemic supply In common, for a drug to be absorbed it must be in answer (molecularly dispersed). Because the amount of liquid that could be administered intranasally is comparatively low (25 �L to 200 �L), drugs with low aqueous solubility and/or those for which a high dose is required could be problematic. Such issues can be overcome by formulation of the drug as a suspension or powder (generally with particles within the micrometre size range), by which case the drug shall be required to dissolve in the fluid of the nasal cavity earlier than absorption. Epithelial barrier � efflux transporters the absorption of certain medication across the nasal epithelium can be limited by the presence of efflux transporters. This transporter is also expressed by cells throughout the intestine and poses an identical barrier to drugs that are orally administered (see Chapter 19). P-gp is a one hundred seventy kDa glycosylated transmembrane protein found within the apical membranes of the cells. It is in a position to bind a broad variety of hydrophobic and amphiphilic substrates, including sure peptides, to its binding site, which is positioned cytoplasmically on the internal leaflet of the apical cell membrane, and actively pump them from the cell, again into the nasal cavity. Hence medication which are substrates for P-gp might be less well absorbed across the nasal epithelium than their physicochemical properties (molecular dimension, lipophilicity, diploma of ionization) may predict. Active transport/efflux operates towards a focus gradient, is saturable and can be competitively inhibited by other substrates for the binding site. Thus coadministration of an inhibitor of P-gp, corresponding to rifampicin or verapamil, can improve drug absorption. P-gp can additionally be discovered within the olfactory epithelium, at the next concentration 678 Solubility the methods to improve the solubility of a drug can contain (1) number of a special salt kind, (2) modification of its molecular type (including the utilization of a prodrug) and (3) the use of applicable excipients, such as cosolvents, when the drug is formulated (considered later). Prodrugs are sometimes developed to improve the lipophilicity of a drug molecule and hence its absorption throughout a organic membrane. However, in the case of nasal delivery, the principle has been explored to enhance the aqueous solubility of the mother or father drug to allow a clinically relevant dose of the drug to be dissolved in lower than one hundred fifty �L of answer, and has been profitable for several drugs. The prodrug is quickly converted to the active father or mother drug as quickly as it enters the bloodstream. The acceptable selection of the salt form of an ionizable drug can be utilized to increase its aqueous solubility. However, a change in salt type can end result in irritancy to the nasal mucosa and this has to be thought-about when an appropriate counterion is being chosen. The ionized form of the drug also reveals some permeation capability, the diploma of which can be dependent on the nature of the counterion. Lipophilicity/hydrophilicity and molecular dimension Once in answer, lipophilic medication corresponding to propranolol, progesterone and fentanyl are rapidly absorbed from the nasal cavity by the transcellular route and have a nasal bioavailability just like that obtained after intravenous administration (almost 100%). The absorption of hydrophilic (polar) medication occurs through the paracellular route (between the epithelial cells by way of the tight junctions), and the rate and extent of absorption is inversely proportional to the molecular weight of the drug. The paracellular route offers a a lot smaller area for absorption than the transcellular route. Thus the absorption of hydrophilic compounds is way slower than that of lipophilic medication. For each lipophilic and hydrophilic molecules, absorption is comparatively environment friendly for medicine with a molecular mass under 1 kDa but then declines as molecular mass increases. With regard to dose reproducibility from the nasal cavity, dosing is relatively consistent for low molecular weight medication compared with the oral or parenteral routes, whereas for compounds with a excessive molecular weight, similar to peptides and proteins, comparatively excessive variability is exhibited in contrast with injections. Formulation components affecting intranasal systemic delivery the same common formulation concerns apply to drugs formulated for systemic motion as for local action, as indicated by the examples proven in Table 38. However, further methods can be utilized to improve absorption across the nasal epithelium. In essence, the bioavailability of nasally administered medication could be restricted by: � low aqueous solubility; � fast and extensive enzymatic degradation of the drug within the nasal cavity; � brief contact time between the drug and the absorptive epithelium of the turbinates due to mucociliary clearance; and � poor permeation of the drug across the respiratory epithelium. The approaches which were used to overcome these limitations are summarized in Table 38. Increasing aqueous solubility As mentioned already, for a drug to be absorbed, it should normally be in resolution. Drug solubility may be elevated by use of a mixed solvent system or a cosolvent within the formulation. Cyclodextrins (see Chapter 24) are cyclic compounds composed of -D-glucopyranose units. They are inclined to be water soluble because of their hydrophilic/ polar outer surface, however have a hydrophobic/less polar 679 Degree of ionization For medication which are weak acids or bases, the pH of the nasal cavity will have an effect on the diploma of ionization of the drug. In addition, the pH of the formulation itself can alter the native pH, notably if buffered vehicles are used. They are capable of increase the aqueous solubility of lipophilic compounds by forming dynamic inclusion complexes the place the lipophilic a half of the drug molecule is included into the lipophilic central cavity of the cyclodextrin ring. An intranasal formulation containing 17-estradiol solubilized in dimethyl-cyclodextrin (seven glucopyranose units) was obtainable for the treatment of menopausal signs, till it was withdrawn in 2006.
Ephedra Sinica (Ephedra). Prometrium. - Are there any interactions with medications?
- What is Ephedra?
- Are there safety concerns?
- Weight loss. Ephedra can produce modest weight loss when used with exercise and a low fat diet, but it can cause serious side effects, even in healthy people who follow product dosage directions.
- Dosing considerations for Ephedra.
Source: http://www.rxlist.com/script/main/art.asp?articlekey=96822
Cheap prometrium 100mg amexType I glass containers are suitable for many parenteral and nonparenteral merchandise. This glass incorporates alkaline metallic oxides, primarily sodium oxide, and alkaline earth oxides, primarily, calcium oxide, within the glass network. This glass itself has a reasonable hydrolytic resistance because of its chemical composition. However, treatment of the inside floor of the glass container with sulfur dioxide will increase the hydrolytic resistance of the container, because the sulfur dioxide reacts with the oxides discovered on the glass surface. For example, sodium oxide is converted to sodium sulfate, which may then be eliminated by washing of the glass. As talked about already, treatment of the internal floor of glass containers produced with soda�lime� silica glass increases the hydrolytic resistance. The outer surface of glass containers may also be handled to cut back friction and for protection towards abrasion and breakage. Salts within the glass migrate from the physique of the glass and accumulate at its floor. When the paper material weighs 250 g m-2 or extra or is 300 �m or extra in thickness, it is called paperboard. Softwood from spruce, fir, pine and eucalyptus timber is now the commonest source of fibre in papermaking, although bagasse (from sugar cane), cotton, straw, flax, bamboo, jute, hemp, grass, esparto, rags and sisal have also been used. To produce paper, cellulose fibre is extracted from the wooden by the pulping of the latter (mechanically and/or chemically). The pulp is then mechanically handled to break down any fibre bundles, to hydrate and break up the surface of the fibres, and this is then bleached if desired. A variety of nonfibrous components are added to the treated pulp to control water and ink permeation (rosins), to enhance strength (starches, gums, resins) and to improve the optical brightness and printing qualities (clay, talc, titanium dioxide). The latter is pressed between a number of stacks of heavy rollers, which smooth out the floor of the paper and make it more appropriate for printing. A number of coatings may then be utilized to the paper to additional improve its floor properties, similar to to cut back its porosity and liquid penetration price (using gelatin, starch, modified rosins or waxes) or increase its opacity, gloss, brightness and printability (using clay, calcium carbonate or titanium dioxide). It can be tailored in hardness and flexibility with respect to the desired container. Both delicate and onerous types of aluminium and tinplate are utilized in pharmaceutical packaging; exhausting material is used for its energy and durability in containers such as aerosol cans, while soft and malleable metal is used to produce collapsible tubes and versatile pouches and because the collar/overcap on parenteral vials with rubber stoppers. Malleability permits the metal collar/overcap to be crimped in place and flexible pouches and steel tubes to be crimped closed. The nature and properties of the completed paper can be controlled, such that paper may be tailor-made for particular applications. For example, its opacity and color can be varied by means of components, and its porosity may be adjusted to permit diffusion of steam for sterilization whereas sustaining a barrier to microorganisms. Laminates are used to produce pharmaceutical packs such as sachets, blister packs, tubes and pouches. An instance is a structure consisting of paper, metallic foil and polythene plies, used for sachet packaging. The paper provides strength, printability and the ability to easily tear the package, the foil supplies a wonderful barrier to gentle, moisture and gases and the polythene supplies warmth sealability. The packaging is taken into account part of the product, and manufacturers must submit giant amounts of information to present that the packaging is protected, is efficacious and performs as claimed. The regulatory our bodies produce steerage documents to assist manufacturers, such as on labelling, patient information leaflets, closures and the testing of containers. Paper does have certain disadvantages, and these result in it not generally getting used on its own within the primary pack. These disadvantages embody: � the shortage of barrier properties against moisture, gases and odours. Repackaging An authentic pack is one which is intended to be distributed on to the affected person without modification apart from the addition of appropriate labelling. Many medicines are packaged by the manufacturer into such packs which could be allotted directly to the affected person. The repacker should be conscious of the problems concerning medication repackaging, such as the potential for errors, the bodily and chemical stability of medicine and medicines, cleanliness, crosscontamination, shelf life of repackaged products, authorized elements, and clear and full labelling. Actions that might end in clearer and safer packaging have been beneficial by numerous researchers and establishments. Examples of fine practice embrace: � selecting drugs names which would possibly be least more doubtless to � � � � � � � � Designing packaging for protected medicine use A quarter of treatment errors � that are common and cause important morbidity, mortality and cost � have been attributed to medicines which have similar names (similar looking and related sounding names) or similar packaging (Emmerton & Rizk, 2012). For example, no less than 15 kids died following vaccination towards measles in northern Syria in 2014, when atracurium was mistakenly used as an alternative of sterile water. Both vials had an analogous look, and the atracurium vials had been incorrectly added to vaccination packs (Pakenham-Walsh & Ana, 2014). Clearly pharmacists and pharmaceutical scientists can play a major half in lowering packaging-related medication errors, given their roles in dispensing and labelling medicines, as well as in the pharmaceutical industry, together with in packaging and advertising products. A health-IoT platform based mostly on the integration of clever packaging, unobtrusive bio-sensor, and clever drugs field. Design for Patient Safety: A Guide to Labelling and Packaging of Injectable Medicines. The degradation of other elements in the formulation, corresponding to antimicrobial preservatives or antioxidants, may also be a important factor. The nature of the degradation merchandise that type within the dosage form may be the factor that limits the shelf lifetime of a product. This may be because the degradation merchandise are toxic; as an example, the antifungal drug flucytosine degrades to fluorouracil, which is cytotoxic. The degradation merchandise might alternatively give the product an unacceptable appearance. In basic, drug molecules have a tendency to not undergo spontaneous chemical degradation; the trigger is usually some other reactive molecule within the dosage type. Often, this is due to the presence of water or molecular oxygen, but the drug may also react with other formulation constituents or react with different molecules of the same drug. Chemical degradation reactions Hydrolysis Hydrolysis is the breaking of a molecular bond by reaction with water. Water is widespread in pharmaceutical merchandise, either as an ingredient or as a contaminant, and hydrolysis reactions are the most common explanation for chemical degradation. Most hydrolysis reactions involve derivatives of carboxylic acids, such as esters and amides, that are regularly found in drug molecules. The carbon of the ester carboxyl group is comparatively electron deficient, owing to bond polarization caused by the adjacent oxygen atoms. Aspirin is merely too unstable to enable the formulation of an aqueous aspirin product with a suitable shelf life. Hydrolysis reactions of esters, amides and associated molecules are catalysed by an acid and by a base. Amides are inclined to be extra secure to hydrolysis than the corresponding esters as a result of the nitrogen atom is much less electronegative than the oxygen atom in the corresponding ester.
Buy genuine prometrium lineBy incorporation of paclitaxel into albumin nanoparticles, the albumin capabilities to coat the paclitaxel and provide colloidal stabilization to the drug. This circumvents both the low solubility and the Cremophor-associated unwanted side effects. Targeting mechanisms of Abraxane the albumin inside the nanoparticle�albumin expertise used in Abraxane serves as more than a solubility-enhancing agent: albumin also promotes lively focusing on of the paclitaxel to tumour cells. The impaired lymphatic drainage at the tumour web site slows drainage of those nanoparticles and macromolecules from the tumour site, resulting within the particles turning into trapped. In the case of Abraxane, a second mechanism has also been associated with the targeting and uptake of the albumin nanoparticles to tumour websites. Within the body, albumin is ready to transport hydrophobic molecules, corresponding to vitamins, hormones and different plasma constituents, throughout the endothelial lining and out of the blood circulation. This is achieved by albumin binding to gp60 albumin receptors found on the floor of vasculature endothelial cells. These gp60 receptors are then answerable for the transport of albumin across blood vessel partitions. The binding of albumin to the gp60 receptors activates the membrane protein caveolin 1. The activation of caveolin 1 subsequently ends in the internalization of the cell membrane and the formation of transcytotic vesicles (known as caveolae). Therefore, Abraxane is prepared to exploit this albumin-activated gp60 transport mechanism to goal the tumour site. After getting into the systemic circulation, the albumin-bound paclitaxel can bind to the gp60 albumin receptors and be carried throughout the endothelial cells via transcytosis in the identical means as albumin. After crossing the endothelial lining, the drug should cross the tumour cell membrane and enter 796 the tumour cells. This can trigger the release of the paclitaxel from the albumin, allowing the free drug to diffuse to the nucleus of tumour cells, initiating cell dying. Inorganic nanoparticles Nanoparticles may be fabricated from inorganic supplies, including metal oxides, metallic sulfides, carbon nanotubes, calcium phosphate and ceramics. These nanoparticles are generally not biodegradable and so have a extra restricted application. Abdoscan is an example (albeit no longer obtainable in Europe) of a metal oxide nanoparticle product. The particles are suspended in viscosity-increasing brokers, similar to starch, to stop aggregation of the particles in vivo. These bilayer membranes could be composed of natural or synthetic amphiphilic lipids, and commonly phospholipids are used for the formulation of liposomes; however, a spread of amphiphilic lipids can be used. Liposomes form when the lipids (which are floor energetic with a hydrophilic head group and a hydrophobic chain(s) at opposing ends of the molecule) are uncovered to an aqueous surroundings. At the appropriate lipid-to-water ratio and temperature, the lipids will organize themselves into bilayer vesicles. Liposomes are capable of carry both water-soluble and lipophilic moieties, with water-soluble drugs being incorporated within the aqueous compartments and lipid-soluble medication being included within the bilayer (similar to the solubilization of medication within the core of micelles). In addition, some medicine and molecules could be adsorbed onto the floor of the liposomes by way of electrostatic interactions. Liposomes can be formulated in a variety of diameters from roughly 30 nm up to a quantity of micrometres, and due to this fact they can be considered as nanotechnology. They are generally easier to prepare in a homogeneous measurement range than different forms of vesicles and are probably the most commonly utilized in clinically accredited products. These vesicles provide a larger aqueous compartment than small unilamellar vesicles. These vesicles have multiple concentric bilayers and are a hundred nm to several micrometres in measurement, relying on their composition and their technique of preparation. Their low aqueous volume (due to the multiple bilayers) reduces their capacity for carrying water-soluble medication. These embody formulations that are prescribed for the therapy of sure cancers. These formulations exploit the flexibility of liposomes to management the pharmacokinetic profile of their integrated drug. Early within the improvement of liposomes as drug supply vehicles it was established that intravenously injected liposomes interact with blood opsonins, which trigger their elimination from the blood circulation at rates which are dictated by vesicle measurement, lipid composition and surface cost. Sparingly soluble octafluoropropane is incorporated inside these constructs Multivesicular vesicles composed of dioleoyl phosphatidylcholine, ldl cholesterol, dipalmitoyl phosphatidylglycerol and triolein. Cytarabine is entrapped within the aqueous compartments of the liposomes Multivesicular liposomes composed of dioleoyl phosphatidylcholine, dipalmitoyl phosphatidylglycerol, cholesterol and triolein. The subunit antigens are incorporated within the bilayer lipid membrane and the vesicles are approximately a hundred and fifty nm in measurement Similar to Epaxal, the virosomes are prepared from phosphatidylcholine. The subunit antigens are integrated inside the bilayer lipid membrane Liposomes approximately a hundred and eighty nm in measurement and composed of egg phosphatidylcholine and ldl cholesterol. Doxorubicin is loaded into the aqueous core of the liposomes, the place it forms a doxorubicin citrate complicated Dimyristoyl phosphatidylcholine and egg phosphatidylglycerol. Alternatively, when administered via the intramuscular route or subcutaneous route, liposomes attain the lymphatic system, together with the local lymph nodes. This provides the opportunity for liposomes to be used for the supply of vaccines. The utility of liposomes in most cancers chemotherapy There are several liposome merchandise designed for the supply of most cancers chemotherapy, and so they can promote improved drug delivery through a variety of various mechanisms. The liposomes within Myocet are roughly one hundred eighty nm in dimension and composed of egg phosphatidylcholine and ldl cholesterol. DaunoXome, a liposome formulation of daunorubicin, can be able to selectively deliver the entrapped drug to tumour websites. DaunoXome liposomes are formulated from excessive transition temperature lipids and cholesterol, which makes the liposome bilayer resistant to opsonization. This, mixed with their small measurement (45 nm), supports extended blood residence times and tumour focusing on. Liposomal delivery of vaccines In addition to the power of liposomes to passively target tumour websites, the pure tendency for liposomes to be taken up by phagocytic cells may be exploited to target cells of the immune system, such as dendritic cells, and thereby improve the supply of antigens. A vary of bilayer vesicle methods have been shown to induce humoral and mobile immunity to a extensive range of antigens. This is especially helpful for the delivery of soluble antigens which have an excellent security profile however are weak at inducing immune responses. There are presently two liposome-type constructs, which are built from viral parts and phospholipids, which might be licensed as vaccine delivery systems (Table 44. These virosomes use viral parts, similar to influenza haemagglutinin, which enhances antigen delivery and concentrating on to the immune cells. Sustained drug launch from liposomes Sustained drug launch, multivesicle liposomes have been developed for clinical use. Both DepoCyte and DepoDur comprise giant multivesicular vesicles which obtain sustained drug release through their gradual clearance from the administration site and their sluggish breakdown. DepoDur is administered as a single epidural injection and can give reduction from postoperative ache for as much as forty eight hours.

Discount prometrium 200mg with amexThey degrade the polysaccharides to produce short-chain fatty acids (acetic, propionic and butyric acids), which lower the luminal pH, and the gases hydrogen, carbon dioxide and methane. Recently there has been a lot curiosity in the exploitation of the enzymes produced by these micro organism with respect to focused drug supply to this area of the gastrointestinal tract. It stretches from the ileocaecal junction to the anus and makes up roughly the final 1. However, the microvilli of the absorptive epithelial cells, the presence of crypts and the irregularly folded mucosae serve to increase the floor area of the colon by 10 to �15 times that of a easy cylinder. The surface area nevertheless Transit of prescription drugs in the gastrointestinal tract As the oral route is the one by which nearly all of pharmaceuticals are administered, it is essential to know how these materials behave during their passage through the gastrointestinal tract. It is understood that the small gut is the major web site of drug absorption, and thus the time a drug is current on this a half of the gastrointestinal tract is extremely important. If sustained-release or controlled-release drug supply systems are being designed, you will need to contemplate factors that can have an result on their behaviour and, in particular, their transit times by way of certain regions of the gastrointestinal tract. In basic, most dosage forms, when taken in an upright place, transit the oesophagus quickly, normally in lower than 15 seconds. Transit by way of the oesophagus relies on both the dosage form and the posture. Adhesion to the oesophageal wall can happen because of partial dehydration on the site of contact and the formation of a gel between the formulation and the oesophagus. Transit of liquids, for example, has at all times been noticed to be rapid, and generally quicker than that of solids. Gastric emptying the time a dosage kind takes to traverse the abdomen is normally termed the gastric residence time, gastric emptying time or gastric emptying price. Gastric emptying of prescribed drugs is highly variable and relies on the dosage kind and the fed/fasted state of the stomach. Normal gastric residence occasions usually vary between 5 minutes and a pair of hours, though for a lot longer instances (>12 h) have been recorded, notably for giant single dosage items. Phase I is a comparatively inactive interval of 40 to 60 minutes with solely uncommon contractions occurring. The cycle repeats itself every 2 hours until a meal is ingested and the fed state or motility is initiated. The proximal part of the abdomen relaxes to obtain meals, and gradual contractions of this area transfer the contents distally. Peristalsis � contractions of the distal a part of the abdomen � serves to mix and break down food particles and move them in the course of the pyloric sphincter. The pyloric sphincter permits liquids and small meals particles to empty whereas other material is retropulsed into the antrum of the abdomen and is caught up by the next peristaltic wave for additional dimension reduction earlier than emptying. Many components influence gastric emptying, in addition to the sort of dosage type and the presence of meals. These embody posture, the composition of the food and the effect of medicine and disease state. In general, food, significantly fatty foods, delays gastric emptying and hence the absorption of drugs. Therefore a drug is likely to attain the small gut most rapidly if it is administered with water to a affected person whose abdomen is empty. Small intestinal transit There are two primary types of intestinal movement � propulsive and mixing. The propulsive movements primarily decide the intestinal transit fee and hence the residence time of the drug or dosage form within the small intestine. As that is the primary web site of absorption in the gastrointestinal tract for many medication, the small intestinal transit time. Small intestinal transit is generally thought of to be between 3 and 4 hours, though each sooner and slower transits have been measured. Small intestinal residence time is especially essential for: � dosage varieties that release their drug slowly. Contractile activity in the colon may be divided into two main sorts: � Propulsive contractions or mass actions which might be associated with the aboral (away from the mouth) motion of contents. Segmental contractions are led to by contraction of the round muscle and predominate, whereas the propulsive contractions, which are as a end result of contractions of the longitudinal muscle, occur only three to four occasions daily in regular individuals. Colonic transit is thus characterized by short bursts of exercise followed by long intervals of stasis. Motility and transit are highly influenced by defecation time; each the frequency of defecation and the chance of being included in a defecation occasion. The drug needs to remain in solution, not turn into certain to meals or different materials within the gastrointestinal tract and never precipitate. It needs to be chemically stable in order to face up to the pH of the gastrointestinal tract and it should be immune to enzymatic degradation in the lumen. The drug then must diffuse across the mucous layer with out binding to it, throughout the unstirred water layer and subsequently across the gastrointestinal membrane, its primary cellular barrier. After passing by way of this mobile barrier, the drug encounters the liver and all its metabolizing enzymes before it reaches the systemic circulation. Any of these limitations can stop some or the entire drug reaching the systemic circulation and may subsequently have a detrimental effect on its bioavailability. Environment within the lumen the surroundings throughout the lumen of the gastrointestinal tract has a significant impact on the speed and extent of drug absorption. Gastrointestinal pH the pH of fluids varies considerably along the length of the gastrointestinal tract. Depending on the size of the meal, the gastric pH returns to the decrease fasted-state values inside 2 to three hours. Thus solely a dosage type ingested with or quickly after a meal will encounter these larger pH values. This could also be an necessary consideration in phrases of the chemical stability of a drug or in attaining drug dissolution or absorption. Intestinal pH values are larger than gastric pH values owing to the neutralization of the gastric acid by bicarbonate ions secreted by the pancreas into the small gut. There is a gradual rise in pH along the length of the small intestine from the duodenum to the ileum. The pH drops again within the colon because the bacterial enzymes, that are localized in the colonic region, break down undigested carbohydrates into short-chain fatty acids; this lowers the pH in the colon to roughly 6. The gastrointestinal pH might affect the absorption of medication in a big selection of methods. Chemical degradation due to pH-dependent hydrolysis can happen in the gastrointestinal tract. The results of this instability is incomplete bioavailability, as solely a fraction of the administered dose reaches the systemic circulation in the form of intact drug. The extent of degradation of penicillin G (benzylpenicillin), the first of the penicillins, after oral administration is dependent upon its residence time in the stomach and the gastric pH.
References - McAchran SE, Lesani OA, Resnick MI: Radiofrequency ablation of renal tumors: past, present, and future, Urology 66(5 Suppl):15n22, 2005.
- Bremholm Rasmussen T, Ingerslev HJ, Hostrup H: Bilateral spontaneous descent of the testis after the age of 10: subsequent effects on fertility, Br J Surg 75(8):820n823, 1988.
- Shuch B, Singer EA, Bratslavsky G: The surgical approach to multifocal renal cancers: hereditary syndromes, ipsilateral multifocality, and bilateral tumors, Urol Clin North Am 39(2):133n148, v, 2012. Shuch B, Vourganti S, Ricketts CJ, et al: Defining early-onset kidney cancer: implications for germline and somatic mutation testing and clinical management, J Clin Oncol 32(5):431n437, 2014.
|
|